

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Given the redox reaction:
cr3+ + al--> cr + al3+
as the reaction takes place, there...
cr3+ + al--> cr + al3+
as the reaction takes place, there...
Questions in other subjects:

Biology, 18.12.2019 10:31

History, 18.12.2019 10:31

Mathematics, 18.12.2019 10:31



History, 18.12.2019 10:31


Mathematics, 18.12.2019 10:31


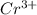
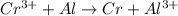
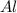 to
to 

