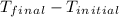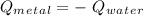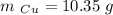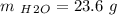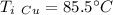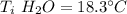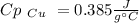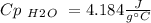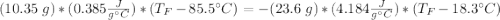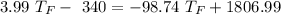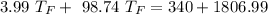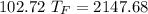Chemistry, 23.08.2019 03:00 carafaith02
A10.35g piece of copper metal was heated to 85.5 degrees c and then placed into 23.6g of water. the initial temperate of the water was 18.3 degrees c. what was the final temperature?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kkruvc
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1


Chemistry, 22.06.2019 21:30, MJyoungboy
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
A10.35g piece of copper metal was heated to 85.5 degrees c and then placed into 23.6g of water. the...
Questions in other subjects:



Physics, 12.07.2019 19:00

Mathematics, 12.07.2019 19:00


History, 12.07.2019 19:00




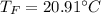
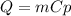 ΔT
ΔT