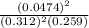Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2


Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
An experiment is conducted using nitrogen monoxide and bromine reacted to form nitrosyl bromide. cal...
Questions in other subjects:

Mathematics, 23.11.2019 14:31

Mathematics, 23.11.2019 14:31






Mathematics, 23.11.2019 14:31

Mathematics, 23.11.2019 14:31

![\frac{[B]^b}{[A]^a}](/tpl/images/0368/3891/8cad0.png)
![\frac{[NOBr]^2}{[NO] [Br2]}](/tpl/images/0368/3891/39eea.png) =
=