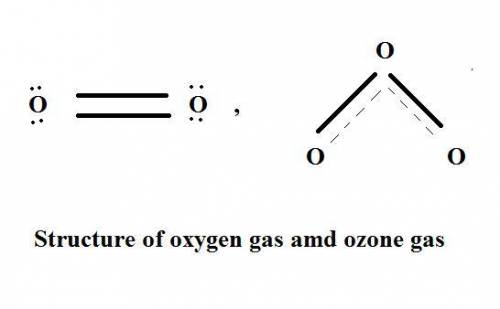
Which statement explains why ozone gas, o3, and oxygen gas, o2, have different properties?
(1...

Chemistry, 30.09.2019 10:30 sharnisefrazier
Which statement explains why ozone gas, o3, and oxygen gas, o2, have different properties?
(1) they are formed from different elements.
(2) they have different molecular structures.
(3) they have different oxidation numbers.
(4) they have different electronegativities.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Questions in other subjects:










 (Paramagnetic)
(Paramagnetic) (diamagnetic)
(diamagnetic)