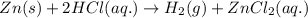Chemistry, 24.08.2019 05:20 caprisun1491
A1.0-gram strip of zinc is reacted with hydrochloric acid in a test tube. the unbalanced equation below represents the reaction.
zn(s) + hcl(aq) ==> h2(g) + zncl2(aq)
balance the equation in your answer booklet for the reaction of zinc and hydrochloric acid, using the smallest whole-number coefficients. [1]
zn(s) + hcl(aq) ==> h2(g) + zncl2(aq)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
A1.0-gram strip of zinc is reacted with hydrochloric acid in a test tube. the unbalanced equation be...
Questions in other subjects:


Computers and Technology, 01.04.2020 19:25


Mathematics, 01.04.2020 19:25






Biology, 01.04.2020 19:25