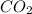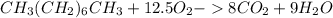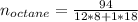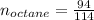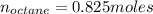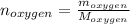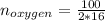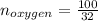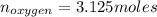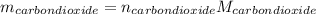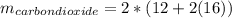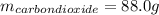Chemistry, 30.08.2019 16:20 deadpoolcorvettehats
Liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 94. g of octane is mixed with 100. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to 3 significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 00:00, ahmedeldyame
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
Liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and...
Questions in other subjects:


Mathematics, 22.07.2019 13:30

Computers and Technology, 22.07.2019 13:30






Biology, 22.07.2019 13:30