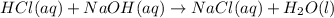Chemistry, 29.01.2020 00:51 sunshine52577oyeor9
What are the products of this chemical equation? hcl(aq) + naoh(aq) → nacl(aq) + h2(g) + o2(g) nah(aq) + cl2(aq) + h2(g) + o2(g) cl2(g) + h2(g) + o2(g) nacl(aq) + h2o(l)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, gilbert325
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
What are the products of this chemical equation? hcl(aq) + naoh(aq) → nacl(aq) + h2(g) + o2(g) nah(...
Questions in other subjects:


Mathematics, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

Computers and Technology, 05.02.2021 01:00




Biology, 05.02.2021 01:00