Chemistry, 03.01.2020 10:31 pikapika24
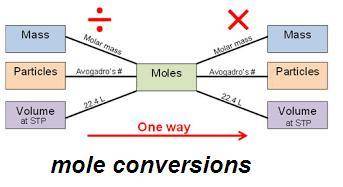
He fuel used in many disposable lighters is liquid butane, c4h10. butane has a molecular weight of 58.1 grams in one mole. how many carbon atoms are in 1.50 g of butane?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, psychocatgirl1
Write the overall equation for the reaction for lithium battery
Answers: 2


Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
He fuel used in many disposable lighters is liquid butane, c4h10. butane has a molecular weight of 5...
Questions in other subjects:








Physics, 05.12.2019 04:31

English, 05.12.2019 04:31

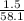 = 0.0258
= 0.0258