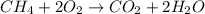Chemistry, 11.10.2019 10:50 rebecca7415
Methane (ch4) is the main component of natural gas. it is burned for fuel in a combustion reaction. the unbalanced combustion reaction for methane is shown below. ch4 + o2 co2 + h2o + heat when the reaction is balanced, how many carbon dioxide molecules are produced for every methane molecule burned?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 07:30, zamirareece17
1. list three scientific reasons cockroaches may fly.
Answers: 1

Chemistry, 22.06.2019 21:00, ciel8809
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
Methane (ch4) is the main component of natural gas. it is burned for fuel in a combustion reaction....
Questions in other subjects:

Mathematics, 09.12.2020 02:30


Engineering, 09.12.2020 02:30

History, 09.12.2020 02:30

Mathematics, 09.12.2020 02:30



History, 09.12.2020 02:30

Social Studies, 09.12.2020 02:30