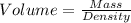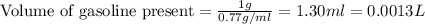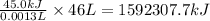Chemistry, 02.09.2019 01:00 cashkidd2200
Agram of gasoline produces 45.0kj of energy when burned. gasoline has a density of 0.77/gml . how would you calculate the amount of energy produced by burning 46.l of gasoline? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 23.06.2019 00:30, joshsmith2022
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 02:00, raulflores01
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
Agram of gasoline produces 45.0kj of energy when burned. gasoline has a density of 0.77/gml . how wo...
Questions in other subjects:

English, 08.10.2020 19:01

Mathematics, 08.10.2020 19:01

English, 08.10.2020 19:01



Social Studies, 08.10.2020 19:01