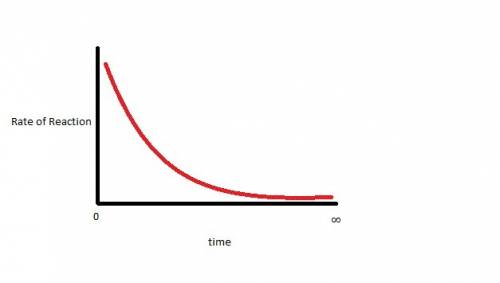
Dinitrogen tetroxide decomposes to produce nitrogen dioxide gas. when dinitrogen tetroxide is sealed in an evacuated glass container, the closed system eventually reaches dynamic equilibrium. which statement describes the graph of the rate of the forward reaction over time? it starts high and gradually decreases until it levels out above zero. it starts high and gradually decreases until it reaches a rate of zero. it starts low and gradually increases until it levels out at a rate above zero. it starts low and gradually increases until it reaches a maximum rate.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, ajaydonlee
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3
You know the right answer?
Dinitrogen tetroxide decomposes to produce nitrogen dioxide gas. when dinitrogen tetroxide is sealed...
Questions in other subjects:



English, 28.01.2021 19:10


Mathematics, 28.01.2021 19:10

English, 28.01.2021 19:10

Chemistry, 28.01.2021 19:10


Mathematics, 28.01.2021 19:10