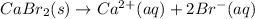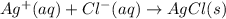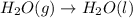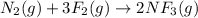Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 23.06.2019 01:30, kaitie60
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
For which of the following processes would you expect there to be an increase in entropy? ag+(aq) +...
Questions in other subjects:


English, 31.03.2021 18:10






Chemistry, 31.03.2021 18:20


History, 31.03.2021 18:20