
Chemistry, 23.09.2019 05:30 montgomerykarloxc24x
Neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic mass of 40.0 g/mol. how would the number of atoms in a 1.0 mol sample of neon compare to the number of atoms in a 4.0 mol sample of argon

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, sotoamerica0814
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
Neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic mass of 40.0 g/mo...
Questions in other subjects:

Biology, 03.08.2019 19:50

Biology, 03.08.2019 19:50

Mathematics, 03.08.2019 19:50







Mathematics, 03.08.2019 19:50

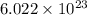 number of atoms.
number of atoms.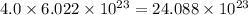 number of atoms
number of atoms

