
Chemistry, 03.10.2019 02:30 kitttimothy55
Consider the reaction.
mc028-1.jpg
which statement is true at stp? (the atomic mass of zn is 65.39 u.)
2 mol of hcl produce 1 l of h2 gas.
1 l of zn produces 1l of h2 gas.
65.39 g of zn produce 22.4 l of h2 gas.
1 l of hcl produces 1 mol of h2 gas.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Consider the reaction.
mc028-1.jpg
which statement is true at stp? (the ato...
mc028-1.jpg
which statement is true at stp? (the ato...
Questions in other subjects:










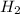 gas.
gas.


