

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, brookemcelhaney
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

You know the right answer?
Consider this reaction: zn(s) + 2hcl(aq) zncl2(aq) + h2(g). which change could decrease the rate of...
Questions in other subjects:

Mathematics, 16.01.2020 14:31




Chemistry, 16.01.2020 14:31

Mathematics, 16.01.2020 14:31

History, 16.01.2020 14:31

English, 16.01.2020 14:31


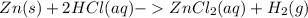
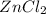 decreases when:
decreases when: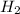 increases.
increases.

