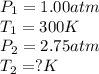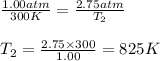Aflask of fixed volume contains 1.00 mole of gaseous carbon dioxide and 88.0 g of solid carbon dioxide. the original pressure and temperature in the flask is 1.00 atm and 300. k. all of the solid carbon dioxide sublimes. the final pressure in the flask is 2.75 atm. what is the final temperature in kelvins? assume the solid carbon dioxide takes up negligible volume.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1


Chemistry, 23.06.2019 01:30, kcarstensen59070
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
Aflask of fixed volume contains 1.00 mole of gaseous carbon dioxide and 88.0 g of solid carbon dioxi...
Questions in other subjects:


Mathematics, 23.02.2021 18:10

Social Studies, 23.02.2021 18:10



Mathematics, 23.02.2021 18:10

Mathematics, 23.02.2021 18:10

Mathematics, 23.02.2021 18:10

Computers and Technology, 23.02.2021 18:10

Mathematics, 23.02.2021 18:10

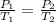 (at constant volume)
(at constant volume)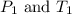 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.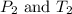 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.