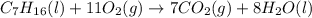What do the symbols in parenthesis indicate?
c7h16 (l) + 11o2 (g) 7co2 (g) + 8h2o (l)
a...

Chemistry, 29.12.2019 07:31 madelyngv97
What do the symbols in parenthesis indicate?
c7h16 (l) + 11o2 (g) 7co2 (g) + 8h2o (l)
a the physical state of each reactant and product
b the catalyst used in the reaction
c the number of atoms of each reactant and product
d the unit of reactant and product
e the rate of the chemical reaction

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1



Chemistry, 23.06.2019 04:31, 1Angel2Got3Brains
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Questions in other subjects:

Biology, 26.07.2019 13:30


Mathematics, 26.07.2019 13:30





Geography, 26.07.2019 13:30

Business, 26.07.2019 13:30