Chemistry, 01.10.2019 17:00 jet0120996
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2mg(s) + o2(g) ? 2mgo(s) when 4.00 g of magnesium burns, the theoretical yield of magnesium oxide is

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1



Chemistry, 22.06.2019 17:30, ander67061
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2mg(s) + o2(g) ? 2mg...
Questions in other subjects:

Mathematics, 24.03.2020 21:26


History, 24.03.2020 21:26

Mathematics, 24.03.2020 21:26

Mathematics, 24.03.2020 21:26

History, 24.03.2020 21:26



Arts, 24.03.2020 21:26

English, 24.03.2020 21:26

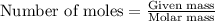 .....(1)
.....(1)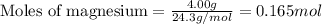
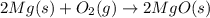
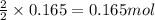 of magnesium oxide
of magnesium oxide