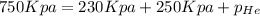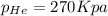Chemistry, 02.10.2019 16:40 Blahdjwj7073
The total pressure inside a vessel containing a mixture of neon, argon, and helium gases is 750 kpa. the partial pressure of neon is 230 kpa, and the partial pressure of argon is 250 kpa. what is the partial pressure of helium?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

You know the right answer?
The total pressure inside a vessel containing a mixture of neon, argon, and helium gases is 750 kpa....
Questions in other subjects:



English, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01



Social Studies, 20.10.2020 22:01

English, 20.10.2020 22:01

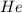 is, 270 Kpa
is, 270 Kpa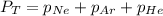
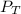 = total partial pressure = 750 Kpa
= total partial pressure = 750 Kpa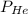 = partial pressure of helium = ?
= partial pressure of helium = ?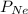 = partial pressure of neon = 230 Kpa
= partial pressure of neon = 230 Kpa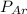 = partial pressure of argon = 250 Kpa
= partial pressure of argon = 250 Kpa