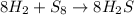Consider the balanced equation below. 8h2 + s8 mc006-1.jpg 8h2s based on the mole ratios, what can most likely be predicted? 1 mol of hydrogen will react with 1 mol of sulfur. 8 mol of hydrogen will react with 1 mol of sulfur. 8 mol of hydrogen will react with 8 mol of sulfur. 16 mol of hydrogen will react with 8 mol of sulfur.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, sandersmakaylaovq5vu
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3


Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3
You know the right answer?
Consider the balanced equation below. 8h2 + s8 mc006-1.jpg 8h2s based on the mole ratios, what can m...
Questions in other subjects:

Mathematics, 22.03.2021 18:20

Social Studies, 22.03.2021 18:20



Mathematics, 22.03.2021 18:20


History, 22.03.2021 18:20


Chemistry, 22.03.2021 18:20