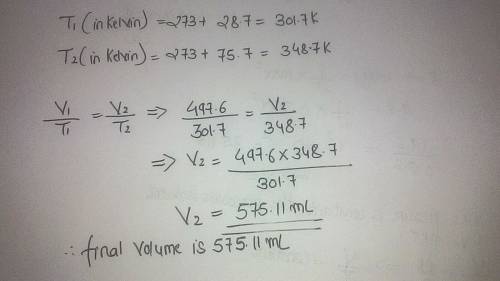Chemistry, 31.01.2020 21:53 gatorboy1788
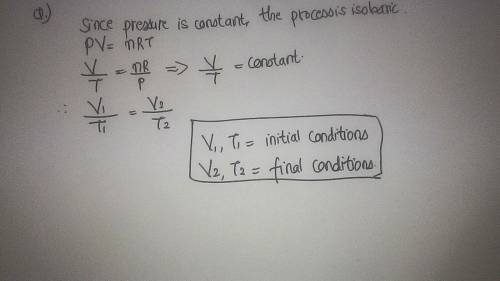
The volume of a gas is 497.6 ml at 28.7c. if the temperature is increased to 75.7c without changing the pressure, what is the new volume of the gas

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, sotoamerica0814
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
The volume of a gas is 497.6 ml at 28.7c. if the temperature is increased to 75.7c without changing...
Questions in other subjects:

History, 05.03.2021 22:00

Business, 05.03.2021 22:00

Chemistry, 05.03.2021 22:00



Physics, 05.03.2021 22:00



Mathematics, 05.03.2021 22:00

History, 05.03.2021 22:00