
Chemistry, 02.10.2019 10:10 terrasami2330
In the reaction mg(s) + 2hcl(aq) → h2(g) + mgcl2(aq), how many moles of hydrogen gas will be produced from 250.0 milliliters of a 3.0 m hcl in an excess of mg? 0.75 moles 0.38 moles 3.0 moles 1.5 moles

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, maddietomlinson113
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a. inner transition b. noble gases c. representative d. transition
Answers: 2

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 23.06.2019 00:00, PlzNoToxicBan
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
You know the right answer?
In the reaction mg(s) + 2hcl(aq) → h2(g) + mgcl2(aq), how many moles of hydrogen gas will be produce...
Questions in other subjects:


Mathematics, 30.07.2019 13:30


History, 30.07.2019 13:30





Mathematics, 30.07.2019 13:30

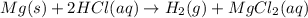
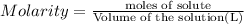
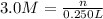
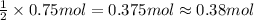 of hydrogen gas.
of hydrogen gas.


