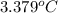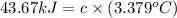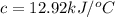Chemistry, 26.09.2019 22:40 kraigstlistt
At constant volume, the heat of combustion of a particular compound, compound a, is –3568.0 kj/mol. when 1.411 g of compound a (molar mass = 115.27 g/mol) was burned in a bomb calorimeter, the temperature of the calorimeter (including its contents) rose by 3.379 °
c. using this data, what is the heat capacity (calorimeter constant) of the calorimeter?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3



Chemistry, 23.06.2019 11:30, angelicar1160
Which of the following is a property of an acid solution? a. slippery to the touch b. ph less than 7 c. turns red litmus paper blue d. bitter taste
Answers: 1
You know the right answer?
At constant volume, the heat of combustion of a particular compound, compound a, is –3568.0 kj/mol....
Questions in other subjects:


SAT, 26.05.2021 17:20

Mathematics, 26.05.2021 17:20

Chemistry, 26.05.2021 17:20

English, 26.05.2021 17:20



Social Studies, 26.05.2021 17:20


Mathematics, 26.05.2021 17:20

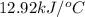
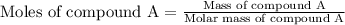
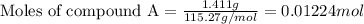
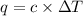
 = change in temperature =
= change in temperature =