

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1


Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Give the theoretical yield, in moles, of co2 from the reaction of 4.00 moles of c8h18 with 4.00 mole...
Questions in other subjects:

Mathematics, 04.02.2020 01:55



History, 04.02.2020 01:55


History, 04.02.2020 01:55

English, 04.02.2020 01:55

History, 04.02.2020 01:55


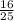 x 4 ) moles of CO₂
x 4 ) moles of CO₂ 

