
Chemistry, 29.08.2019 14:30 avahrider1
The pressure of 5.00 l of gas increases from 1.50 atm to 1240 mmhg. what is the final volume of the gas, assuming constant temperature?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, brookekolmetz
How many orbitals does the p sub shell container
Answers: 3



Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1
You know the right answer?
The pressure of 5.00 l of gas increases from 1.50 atm to 1240 mmhg. what is the final volume of the...
Questions in other subjects:

History, 11.10.2020 22:01

Mathematics, 11.10.2020 22:01


Social Studies, 11.10.2020 22:01



Mathematics, 11.10.2020 22:01

English, 11.10.2020 22:01


Mathematics, 11.10.2020 22:01



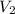 as required, including the conversion from mmHg to atm for the final pressure:
as required, including the conversion from mmHg to atm for the final pressure:


