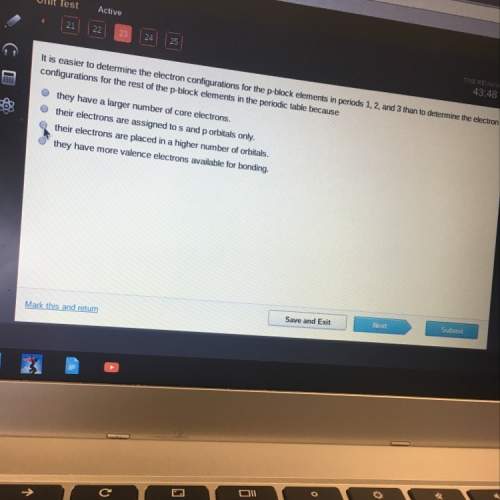
A. they have a larger number of core electrons
b. their electrons are assigned to s and p orbi...

Chemistry, 25.11.2019 09:31 faithyholcomb
A. they have a larger number of core electrons
b. their electrons are assigned to s and p orbitals only.
c. their electrons are placed in a higher number of orbitals.
d. they have more valence electrons available for bonding

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:30, Averybeam300
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

You know the right answer?
Questions in other subjects:

Chemistry, 19.12.2020 04:30

English, 19.12.2020 04:30




Social Studies, 19.12.2020 04:30

Mathematics, 19.12.2020 04:30


Mathematics, 19.12.2020 04:30

English, 19.12.2020 04:30



