
Chemistry, 02.09.2019 19:00 deanlmartin
A50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a coffee-cup calorimeter filled with water. the calorimeter and the water have a combined mass of 250.0 g and an overall specific heat of 1.035 cal/g•°c. the initial temperature of the calorimeter is 10.00°c. the system reaches a final temperature of 11.08°c when the metal is added.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2


Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2

Chemistry, 22.06.2019 21:30, MJyoungboy
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
A50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a coffee-cup calorime...
Questions in other subjects:


Mathematics, 30.10.2019 06:31

Mathematics, 30.10.2019 06:31

Mathematics, 30.10.2019 06:31




Health, 30.10.2019 06:31


Geography, 30.10.2019 06:31

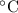 .
. specific heat capacity
specific heat capacity 


