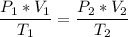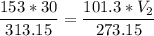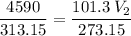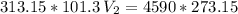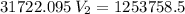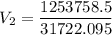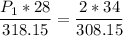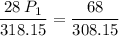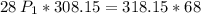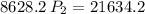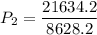Chemistry, 05.10.2019 09:01 zhellyyyyy
The volume of a gas-filled balloon is 30.0 l at 40 °c and 153 kpa pressure. what volume will the balloon have at standard temperature and pressure (273.15 k and 101.3 kpa)?
a. 17.3 l
b. 23.7 l
c. 39.5 l
d. 51.9 l
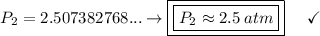
a gas that has a volume of 28 liters, a temperature of 45 °c, and an unknown pressure, has its volume increased to 34 liters and its temperature decreased to 35 °c. if i measure the pressure after the change to be 2.0 atm, what was the original pressure of the gas?
a. 1.5 atm
b. 1.7 atm
c. 2.8 atm
d. 2.5 atm

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 22.06.2019 14:20, montanolumpuy
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d. lytic
Answers: 1

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
The volume of a gas-filled balloon is 30.0 l at 40 °c and 153 kpa pressure. what volume will the bal...
Questions in other subjects: