
Chemistry, 18.11.2019 07:31 esdoles3865
If 5.65 grams of zinc metal react with 21.6 grams of silver nitrate, how many grams of silver metal can be formed and how many grams of the excess reactant will be left over when the reaction is complete? show all of your work.
unbalanced equation: zn + agno3 zn(no3)2 + ag

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 22:00, jlegrand9098
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
If 5.65 grams of zinc metal react with 21.6 grams of silver nitrate, how many grams of silver metal...
Questions in other subjects:

Mathematics, 05.12.2019 09:31




History, 05.12.2019 09:31


Computers and Technology, 05.12.2019 09:31

History, 05.12.2019 09:31

Mathematics, 05.12.2019 09:31

Mathematics, 05.12.2019 09:31

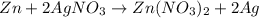
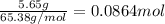
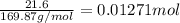
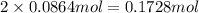 silver nitrate
silver nitrate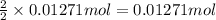 of silver metal
of silver metal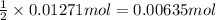 of zinc
of zinc

