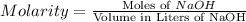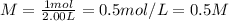Chemistry, 29.01.2020 02:55 19thomasar
Achemist uses 0.25 l of 2.00 m h2so4 to completely neutralize a 2.00 l of solution of naoh. the balanced chemical equation of the reaction is given below. 2naoh + h2so4 na2so4 + 2h2o what is the concentration of naoh that is used?

Answers: 1


Other questions on the subject: Chemistry


You know the right answer?
Achemist uses 0.25 l of 2.00 m h2so4 to completely neutralize a 2.00 l of solution of naoh. the bala...
Questions in other subjects:


History, 17.04.2021 23:40




World Languages, 17.04.2021 23:50



Mathematics, 17.04.2021 23:50

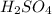 in 0.25 L of 2.00 M solution:
in 0.25 L of 2.00 M solution: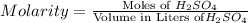
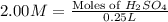
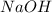 in 2.00 L of an unknown Molarity :
in 2.00 L of an unknown Molarity :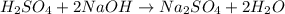
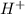 ions.
ions.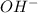 ions.
ions.