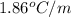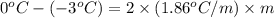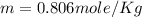Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3



Chemistry, 23.06.2019 13:00, kayleegeise
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1
You know the right answer?
What is the molality of a solution of water and kcl if the freezing point of the solution is –3°c?...
Questions in other subjects:


Mathematics, 06.05.2021 21:30

Mathematics, 06.05.2021 21:30




Mathematics, 06.05.2021 21:30

Mathematics, 06.05.2021 21:30


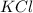 will be,
will be,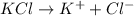
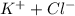 = 1 + 1 = 2
= 1 + 1 = 2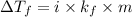
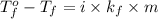
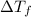 = change in freezing point
= change in freezing point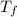 = temperature of solution =
= temperature of solution = 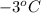
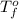 = temperature of pure water =
= temperature of pure water = 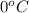
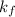 = freezing point constant =
= freezing point constant =