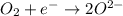Chemistry, 20.09.2019 14:00 luciaaviles3
How many moles of electrons are required to reduce one mole of oxygen gas (o2) to two moles of oxygen ions (
2 e-
4 e-
6 e-
8 e-

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1
You know the right answer?
How many moles of electrons are required to reduce one mole of oxygen gas (o2) to two moles of oxyge...
Questions in other subjects:

Mathematics, 17.07.2020 01:01



Mathematics, 17.07.2020 01:01



Mathematics, 17.07.2020 01:01

English, 17.07.2020 01:01