
Brie is studying the law of conservation of mass at school, so she decides to test it. first, she measures and records the mass of 1 teaspoon of baking soda and 1 tablespoon of vinegar. then, she combines the two substances and observes what happens. she notices that the mixture bubbles vigorously. after the mixture stops bubbling, she measures and records the mass of it. when she examines her data, she notices that the mass of the mixture is less than the mass of the vinegar and baking soda added together. brie is confused because it appears as if mass is not conserved.
which statement best explains why the mass of the reactants in brie's experiment was greater than the mass of the products?
a. some of the products from the experiment were spilled before their mass was determined.
b. some of the products formed from the experiment escaped to the surroundings as a gas.
c. some of the products from the experiment were destroyed by the vigorous bubbling.
d. some of the products formed from the experiment were lower in density.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:00, daniel1480
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced. be sure your answer has the correct number of significant digits.
Answers: 2
You know the right answer?
Brie is studying the law of conservation of mass at school, so she decides to test it. first, she me...
Questions in other subjects:

History, 20.10.2020 02:01


Mathematics, 20.10.2020 02:01



History, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01



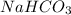 (Base)
(Base)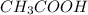 (Acid)
(Acid)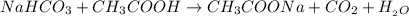
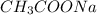 = Sodium Acetate (Salt)
= Sodium Acetate (Salt)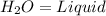 =Water
=Water 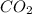 =Carbon dioxide=Gas
=Carbon dioxide=Gas

