
Achemist places a 100 g cube of each of the metals listed in the table on a separate hot plate. all of the hot plates are identical. each hot plate is set on medium heat, and all of the cubes have the same initial temperature. based on the specific heat values in the table, rank the metals in terms of how quickly they will reach the target temperature of 80°c. use "1" to indicate the fastest and "5" to indicate the slowest.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1


You know the right answer?
Achemist places a 100 g cube of each of the metals listed in the table on a separate hot plate. all...
Questions in other subjects:

Mathematics, 23.12.2020 18:40

History, 23.12.2020 18:40


Mathematics, 23.12.2020 18:40

Mathematics, 23.12.2020 18:40

Mathematics, 23.12.2020 18:40

Mathematics, 23.12.2020 18:40



 5 is: Pb, Sn, Cu, Fe, Al.
5 is: Pb, Sn, Cu, Fe, Al.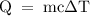
 is the change in temperature.
is the change in temperature.


