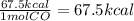Chemistry, 19.09.2019 10:30 destinybonmer
The combustion of 1 mole of co according to the reaction co(g) + ½o2(g) → co2(g) + 67.6 kcal gives off how much heat?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
The combustion of 1 mole of co according to the reaction co(g) + ½o2(g) → co2(g) + 67.6 kcal gives o...
Questions in other subjects:

Mathematics, 08.12.2020 01:50

Chemistry, 08.12.2020 01:50