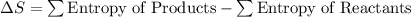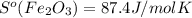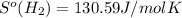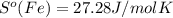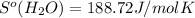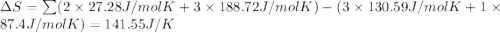Chemistry, 09.11.2019 18:31 straightbarz4643
What is the value for ∆soreaction for the following reaction, given the standard entropy values? fe2o3(s) + 3h2(g) 2 fe(s) + 3h2o(g)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 22:00, Porciabeauty6788
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
What is the value for ∆soreaction for the following reaction, given the standard entropy values? fe...
Questions in other subjects:



History, 21.09.2019 02:00



History, 21.09.2019 02:00


Mathematics, 21.09.2019 02:00