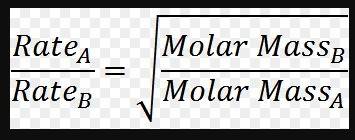In a given sample, hydrogen gas effuses four times faster than the other gas present in the mixture. what is the molar mass and identity of the unknown gas if the molar mass of hydrogen is 2 grams? 16 grams and oxygen 32 grams and oxygen 8 grams and nitrogen 4 grams and helium 64 grams and ozone

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 21:00, thebasedgodchri
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
You know the right answer?
In a given sample, hydrogen gas effuses four times faster than the other gas present in the mixture....
Questions in other subjects:

English, 11.12.2019 06:31


Mathematics, 11.12.2019 06:31

Mathematics, 11.12.2019 06:31




Mathematics, 11.12.2019 06:31

English, 11.12.2019 06:31