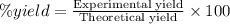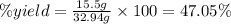One method to produce nitrogen in the lab is to react ammonia with copper (ii) oxide: nh3(
g....

Chemistry, 30.09.2019 12:00 Juliette9525
One method to produce nitrogen in the lab is to react ammonia with copper (ii) oxide: nh3(
g. + cuo(s) cu(s) + h2o(l) + n2(
g. after using 40.0 grams of nh3, 15.5 grams of n2 are produced. what is the percent yield of nitrogen in the reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Questions in other subjects:

Geography, 05.01.2020 15:31




Health, 05.01.2020 15:31

History, 05.01.2020 15:31

Mathematics, 05.01.2020 15:31



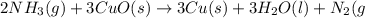
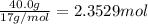
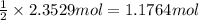 of nitrogen gas
of nitrogen gas