
Chemistry, 24.11.2019 22:31 fortwill05
Rust results from iron’s reaction to oxygen. an iron nail gains mass when it rusts. how does this reaction support the law of conservation of mass?
a. the mass of the rusted nail equals the mass of iron and the oxygen from the air it reacted with to form the rust.
b. the mass of the rusted nail increases because iron attracts more protons from the air.
c. the increased mass of the rusted nail is an exception to the law of conservation of mass since the rusted nail’s mass increases.
d. the increased mass of the rusted nail results from the rearrangement of protons and neutrons within oxygen, according to the law of conservation of mass.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, milkshakegrande101
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
Rust results from iron’s reaction to oxygen. an iron nail gains mass when it rusts. how does this re...
Questions in other subjects:


Biology, 04.07.2020 06:01


History, 04.07.2020 06:01






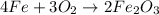
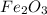 .
.

