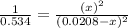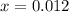Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, bbombard21
Select the atomic models that belong to the same element
Answers: 2

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1
You know the right answer?
For the reaction given below at 700°c, kc = 0.534. h2(g + co2(g h2o(g + co(g calculate the number of...
Questions in other subjects:

Mathematics, 25.06.2019 13:20


Mathematics, 25.06.2019 13:20



Mathematics, 25.06.2019 13:20

Mathematics, 25.06.2019 13:20



Mathematics, 25.06.2019 13:20

 at equilibrium is 0.012 M
at equilibrium is 0.012 M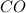 and
and  .
.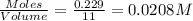
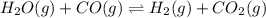
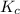 will be,
will be,![K_c=\frac{[H_2][CO_2]}{[H_2O][CO]}](/tpl/images/0263/7107/ded1c.png)
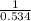 (for reverse reaction).
(for reverse reaction).