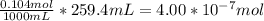Chemistry, 24.09.2019 12:00 divadebbgirl1
When a chemist titrates a standard solution of 168.61 ml of hydrochloric acid (hcl) with 0.104 m sodium hydroxide (naoh) , she finds that it requires 259.4 ml of the base to reach the endpoint of the titration. what is the molarity of the acid solution ?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, eddsworldfrantic
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 21:30, steven0448
An atomic nucleus is composed ofa)protons. b)protons and neutrons. c)protons and electrons. d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
When a chemist titrates a standard solution of 168.61 ml of hydrochloric acid (hcl) with 0.104 m sod...
Questions in other subjects:


History, 06.12.2019 14:31

Mathematics, 06.12.2019 14:31

History, 06.12.2019 14:31

History, 06.12.2019 14:31




English, 06.12.2019 14:31