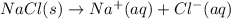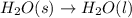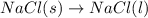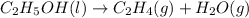Chemistry, 29.09.2019 05:30 rissacoob7862
Which of the following processes would you expect to have a negative value for entropy? nacl(s) na+(aq) + cl- (aq) h2o(s) h2o(l) nacl(s) nacl(l) 2 al(s) + 3br2(l) 2albr3(s) c2h5oh(l) c2h4(g) + h2o(g)

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 02:40, sherlock19
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
Which of the following processes would you expect to have a negative value for entropy? nacl(s) na+...
Questions in other subjects:



Mathematics, 18.09.2021 05:50

Mathematics, 18.09.2021 05:50

Chemistry, 18.09.2021 05:50


Mathematics, 18.09.2021 05:50



Mathematics, 18.09.2021 05:50