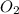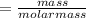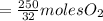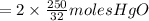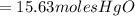Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equation is shown below.
2hgo 2hg + o2
the molar mass of hgo is 216.59 g/mol. the molar mass of o2 is 32.00 g/mol. how many moles of hgo are needed to produce 250.0 g of o2?
3.906
7.813
15.63
73.87

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 10:30, dreamxette3119
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
You know the right answer?
Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equat...
Questions in other subjects:

Physics, 18.07.2019 22:30



SAT, 18.07.2019 22:30



Mathematics, 18.07.2019 22:30

Chemistry, 18.07.2019 22:30

Computers and Technology, 18.07.2019 22:30

Mathematics, 18.07.2019 22:30