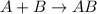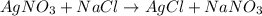What type of reaction does the following equation represent?
agno3 + naci > agci + nano3<...

Chemistry, 31.01.2020 21:53 kymberlyasher
What type of reaction does the following equation represent?
agno3 + naci > agci + nano3
a. synthesis
b. combustion
c. single-displacement reaction
d. double-displacement reaction

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, angelicar1160
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

You know the right answer?
Questions in other subjects:

Biology, 19.05.2020 16:24




Mathematics, 19.05.2020 16:24

Mathematics, 19.05.2020 16:24


Mathematics, 19.05.2020 16:24


History, 19.05.2020 16:24