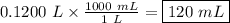Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
The density of benzene at 15 ∘c is 0.8787 g/ml. calculate the mass of 0.1200 l of benzene at this te...
Questions in other subjects:

English, 13.05.2021 18:00



History, 13.05.2021 18:00

Mathematics, 13.05.2021 18:00




Mathematics, 13.05.2021 18:00