
Chemistry, 26.09.2019 11:10 stevensquad638
We have a solution of c5h10o2 of concentration 4 m. find out the volume we need to take from this solution to make a 250 ml solution of concentration 0.5 m.
is my result right? i got 31.25 ml
here's what i did:
m=moles solute / volume (l) solution (m=n/v)
first i calculate the moles of acid needed for a 0.5m, 0.25l solution.
n=m*v = 0.5*0.25 = 0.125 mol acid
now knowing how many moles i need to take, i calculate the volume those moles occupy in the 4m solution.
v=n/m = 0.125/4 = 0.03125 l solution = 31.25 ml
so you need to take 31.25 ml of 4m acid solution and add 218.75 (250*31.25) of water to make a 0.5m, 250 ml solution.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
We have a solution of c5h10o2 of concentration 4 m. find out the volume we need to take from this so...
Questions in other subjects:

History, 10.05.2021 02:10

Mathematics, 10.05.2021 02:10


Mathematics, 10.05.2021 02:10

Mathematics, 10.05.2021 02:10

Geography, 10.05.2021 02:10


Mathematics, 10.05.2021 02:10

Mathematics, 10.05.2021 02:10

Mathematics, 10.05.2021 02:10

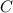 .
. mL solution of concentration
mL solution of concentration 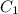 , and we want to know which volume
, and we want to know which volume  of solution to take from the original to do that.
of solution to take from the original to do that.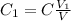 hence
hence 


