
Chemistry, 07.12.2019 19:31 kealinwiley
If all the n2 and h2 are consumed, what volume of nh3, at the same temperature and pressure, will be produced? at a certain temperature and pressure, 1.9 l of n2 reacts with 5.7 l of h2.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 02:50, igraha17
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

Chemistry, 23.06.2019 03:00, kuehlthau03
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 09:00, joelpimentel
The concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point
Answers: 3

Chemistry, 23.06.2019 10:30, lavorisjonesjr1
How much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 3
You know the right answer?
If all the n2 and h2 are consumed, what volume of nh3, at the same temperature and pressure, will be...
Questions in other subjects:

Mathematics, 22.09.2019 08:50





Mathematics, 22.09.2019 08:50



Mathematics, 22.09.2019 08:50

Mathematics, 22.09.2019 08:50

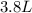 of
of 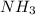
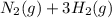 ⇒
⇒ 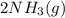
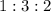 (I)
(I)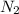 and with 3 moles of
and with 3 moles of 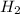 we will obtain 2 moles of
we will obtain 2 moles of 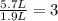 (II)
(II)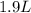 of
of 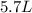 of
of 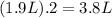 of
of 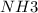 (two times more liters that
(two times more liters that 

