
Chemistry, 21.08.2019 03:30 InnocentSoul
For the reaction ch4 + 2o2 = co2 + 2h2o, how many moles of water are produced from the combustion of 12.5 moles of methane?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 07:00, maryjane8872
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 23.06.2019 04:00, anonymous1813
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
For the reaction ch4 + 2o2 = co2 + 2h2o, how many moles of water are produced from the combustion of...
Questions in other subjects:

Mathematics, 06.09.2020 03:01

Physics, 06.09.2020 03:01



Physics, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01

Business, 06.09.2020 03:01



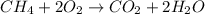
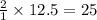 moles of water
moles of water

