Chemistry, 22.12.2019 21:31 amandasantiago2001
Given that the density of water is 0.975 g/ml and that 171 g of sucrose (molar mass: 342.30 g/mol) is dissolved in 512.85 ml of water at 80°c, what is the molality of this solution?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 02:30, micahwilkerson9495
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

You know the right answer?
Given that the density of water is 0.975 g/ml and that 171 g of sucrose (molar mass: 342.30 g/mol)...
Questions in other subjects:


Mathematics, 20.07.2020 21:01

Mathematics, 20.07.2020 21:01


Mathematics, 20.07.2020 21:01

Computers and Technology, 20.07.2020 21:01

Mathematics, 20.07.2020 21:01




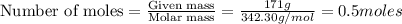
 = weight of solvent in g
= weight of solvent in g