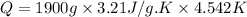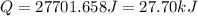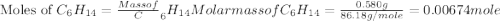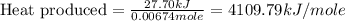Chemistry, 19.11.2019 04:31 billgray2571
Asample of hexane (c6h14) has a mass of 0.580 g. the sample is burned in a bomb calorimeter that has a mass of 1.900 kg and a specific heat of 3.21 j/gik. what amount of heat is produced during the combustion of hexane if the temperature of the calorimeter increases by 4.542 k?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1


Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
Asample of hexane (c6h14) has a mass of 0.580 g. the sample is burned in a bomb calorimeter that has...
Questions in other subjects:

Computers and Technology, 11.02.2020 04:50

Mathematics, 11.02.2020 04:50

English, 11.02.2020 04:50







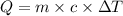
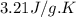
 = change in temperature = 4.542 K
= change in temperature = 4.542 K